F Block Orbital Diagram 32++ Images Result
F Block Orbital Diagram. What block is sn in? 1 for 2, 7 for 8, 25 for 18, and ~64 for 32 along with a few tori.
(refer to the energy level diagram of orbitals in post 28).so, electrons enter 4s orbital first and 3d orbital later. Oxygen (o) electron configuration with full orbital diagram. Which is 2 behind the actual period.
daihatsu copen fuse box 78 ford fuel sending unit wiring ford fuel injector wiring schlage 390g mag lock wiring diagram
F Block Orbital Diagram Wiring Library
Oxygen (o) electron configuration with full orbital diagram. What block is sn in? Draw the molecular orbital diagram of the hydroxyl radical, ho•, clearly labeling the mos as o, o*, t, t*, or nb. Electron would need to have higher potential energy to be in 3s orbital.

Source: 19.mac-happen.de
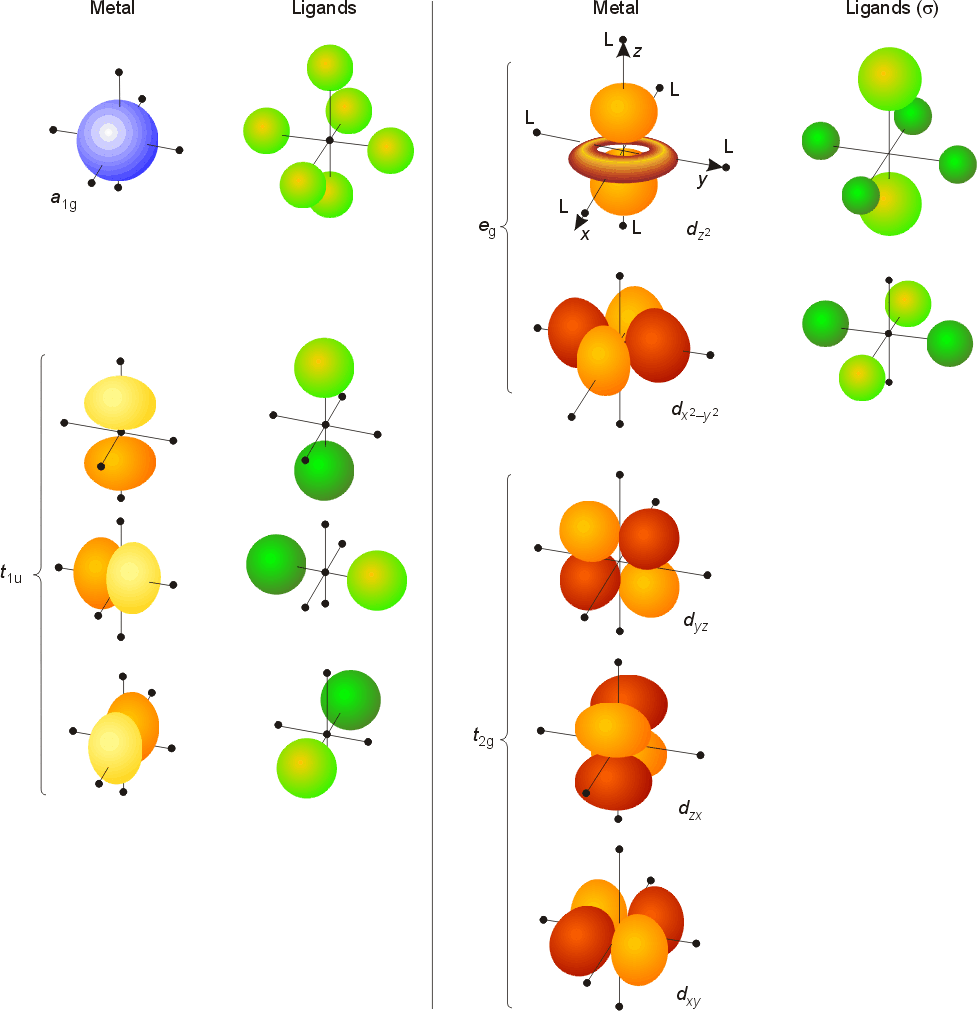
The 3dz² looks like a p orbital wearing a doughnut around its waist. An orbital, their spins must be paired (one must have m s = 1. What groups are in f block? F block fills in period 4: • mo diagrams can be built from group orbitals and central atom orbitals by considering orbital symmetries and energies.

Source: 19.mac-happen.de
(refer to the energy level diagram of orbitals in post 28).so, electrons enter 4s orbital first and 3d orbital later. S p d f orbitals. The lanthanides and actinides form a group that appears almost disconnected from the rest of the periodic table. Oxygen (o) electron configuration with full orbital diagram. Its orbital diagram will keep the noble gas core.

Source: skippingtheinbetween.blogspot.com
What groups are in f block? (refer to the energy level diagram of orbitals in post 28).so, electrons enter 4s orbital first and 3d orbital later. So some barium is an f. About the author randy harvey is the chief of anesthesia and department manager at the florida eye clinic ambulatory surgery center altamonte springs, florida. Orbital blocks commissioned biodigital.

Source: diagramedia.blogspot.com
About the author randy harvey is the chief of anesthesia and department manager at the florida eye clinic ambulatory surgery center altamonte springs, florida. What block is sn in? They'll each occupy separate orbital's because there's um they don't pair up until they have to, and then for the six s, we have two. • the symmetry of group orbitals.
Source: quora.com
Of course, it is, but they have been mathematically generated according to the precepts of • the symmetry of group orbitals is determined by reducing a reducible representation of the orbitals in question. What block is sn in? Principle in addition to listing quantum numbers, n, and subshells, the (\ell)) orbital diagram shows all the different directions and spins of.

Source: youtube.com
This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. S block p block d block from such diagrams, we are able to extract the electronic configurations of elements. Its orbital diagram will keep the noble gas core of zen on, and then we have four f. What block is sn in? 1 for 2, 7.

Source: bibliotekakarpacz.pl
The lanthanides and actinides form a group that appears almost disconnected from the rest of the periodic table. Of course, it is, but they have been mathematically generated according to the precepts of So that's seven possible orbital's 4567 and then we have six s, which has one orbital, so for four f will have six electrons. About the author.

Source: ekerekizul.blogspot.com
N = period # s & p block fill at n. Electron would need to have higher potential energy to be in 3s orbital. What block is sn in? Of course, it is, but they have been mathematically generated according to the precepts of • the symmetry of group orbitals is determined by reducing a reducible representation of the orbitals.

Source: researchgate.net
Orbital blocks commissioned biodigital to create a customized anatomically correct orbital eye model for the purposes of learning ophthalmic blocks. So some barium is an f. This article gives an idea about the electron configuration of oxygen. The atomic mass (which is numerically, a value close to the mass number) is the. (refer to the energy level diagram of orbitals.

Source: mickygurlz.blogspot.com
They can contain up to seven pairs of electrons hence the block occupies fourteen columns in the periodic table. Oxygen (o) electron configuration with full orbital diagram. Orbital diagram in model 3 is higher in energy than ground state because there is an electron in 3s orbital that should be in a 2p orbitals; It explains how to write the.

Source: atkinsjewelry.blogspot.com
So, two electrons in our one s orbital, and one electron in the two s orbital. What groups are in f block? The atomic mass (which is numerically, a value close to the mass number) is the. What block is sn in? The standard atomic mass of oxygen is 15.99903 and its symbol is ‘o’.

Source: drivenheisenberg.blogspot.com
What is the bond order? • the symmetry of group orbitals is determined by reducing a reducible representation of the orbitals in question. Electron would need to have higher potential energy to be in 3s orbital. F block fills in period 4: Which is 2 behind the actual period.

Source: drivenheisenberg.blogspot.com
Electron would need to have higher potential energy to be in 3s orbital. The standard atomic mass of oxygen is 15.99903 and its symbol is ‘o’. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus.the term atomic orbital may also refer to the physical region.
Source: 19.mac-happen.de
Principle in addition to listing quantum numbers, n, and subshells, the (\ell)) orbital diagram shows all the different directions and spins of all electrons. They'll each occupy separate orbital's because there's um they don't pair up until they have to, and then for the six s, we have two electrons, one of each spin, and that's the orbital. They'll each.

Source: untpikapps.com
It explains how to write the orbital diagram n. So that's seven possible orbital's 4567 and then we have six s, which has one orbital, so for four f will have six electrons. This approach is used only when the group orbitals are not obvious by inspection. What groups are in f block? • mo diagrams can be built from.

Source: 19.mac-happen.de
Orbitals are sometimes shown as boxes in a horizontal row. The standard atomic mass of oxygen is 15.99903 and its symbol is ‘o’. Which is 2 behind the actual period. Orbital diagram beryllium has an atomic number of 4, so it has 4 electrons available to place in the orbital diagram. The 3dz² looks like a p orbital wearing a.
Source: diagramedia.blogspot.com
F block fills in period 4: • mo diagrams can be built from group orbitals and central atom orbitals by considering orbital symmetries and energies. This approach is used only when the group orbitals are not obvious by inspection. (refer to the energy level diagram of orbitals in post 28).so, electrons enter 4s orbital first and 3d orbital later. [the.

Source: jalishamav.blogspot.com
It explains how to write the orbital diagram n. Orbital diagram beryllium has an atomic number of 4, so it has 4 electrons available to place in the orbital diagram. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus.the term atomic orbital may also refer.

Source: diagramedia.blogspot.com
So, two electrons in our one s orbital, and one electron in the two s orbital. S block p block d block from such diagrams, we are able to extract the electronic configurations of elements. What block is sn in? What is the bond order? 1 for 2, 7 for 8, 25 for 18, and ~64 for 32 along with.
Source: kabegamiwad.blogspot.com
This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. So that's seven possible orbital's 4567 and then we have six s, which has one orbital, so for four f will have six electrons. So, two electrons in our one s orbital, and one electron in the two s orbital. Oxygen (o) electron configuration with full.